Definition
The reactions where one substance is oxidised and one substance is reduced are called Oxidation-Reduction Reactions or Redox Reactions.
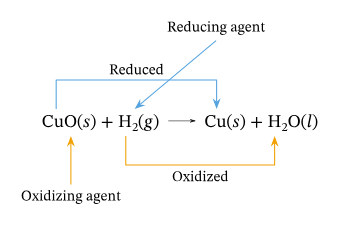
Oxidation
When an object gains Oxygen or loses Hydrogen, it is said to be oxidised.
Reduction
When an object loses Oxygen or gains Hydrogen, it is said to be reduced.
Examples
- Copper Oxide is reduced and Hydrogen is oxidised.
- Zinc Oxide is reduced and Carbon is Oxidised.
Backlinks
Flashcards
What are Oxidation-Reduction or Redox Reactions? ? The reactions where one substance is oxidised and one substance is reduced are called Oxidation-Reduction Reactions or Redox Reactions.
What is reduction?;;When a substance loses Oxygen or gains Hydrogen, it is said to be reduced.
What is oxidation?;;When a substance gains Oxygen or loses Hydrogen, it is said to be oxidised.
What are some examples of Redox Reactions? ?
- Copper Oxide is reduced and Hydrogen is oxidised.
- Zinc Oxide is reduced and Carbon is Oxidised.
Copper Oxide is reduced and Hydrogen is oxidised.;;;
Zinc Oxide is reduced and Carbon is Oxidised.;;;