Definition
The processes are different on the basis of reactivity of the metal. The higher the reactivity, the harder it is to separate the metal
Extraction
Metals of low reactivity
- Usually found in Sulphide Ore.
- Heated in excess air (Roasting) to form metal oxide.
- This metal oxide is then reduced and refined.
Metals of Medium Reactivity
- Found in both Sulphide and Carbonate Ores.
- Sulphide Ores → Roasting
- Carbonate Ores are heated in limited air to form metal oxides.
Metals of High Reactivity
- These are acquired by the electrolysis of the metal’s molten chlorides.
- The metal deposits in Cathode and chlorine is liberated in the anode.
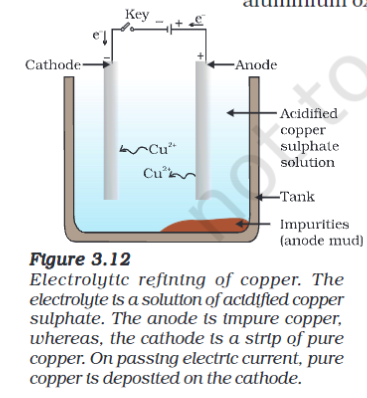
Refining
The obtained metals are often impure. Those are refined using electrolysis.
Backlinks
Flashcards
Extraction of ores in each reactivity ? Metals of low reactivity
- Usually found in Sulphide Ore.
- Heated in excess air (Roasting) to form metal oxide.
- This metal oxide is then reduced and refined. Metals of Medium Reactivity
- Found in both Sulphide and Carbonate Ores.
- Sulphide Ores → Roasting
- Carbonate Ores are heated in limited air to form metal oxides. Metals of High Reactivity
- These are acquired by the electrolysis of the metal’s molten chlorides.
- The metal deposits in Cathode and chlorine is liberated in the anode.