Definition
Carbon has a tendency to form compounds with itself in the form of branches, chains and rings. This is called Catenation.
Structural Isomers
Cyclic (Aromatic) Compounds
These form rings. Example, cyclohexane (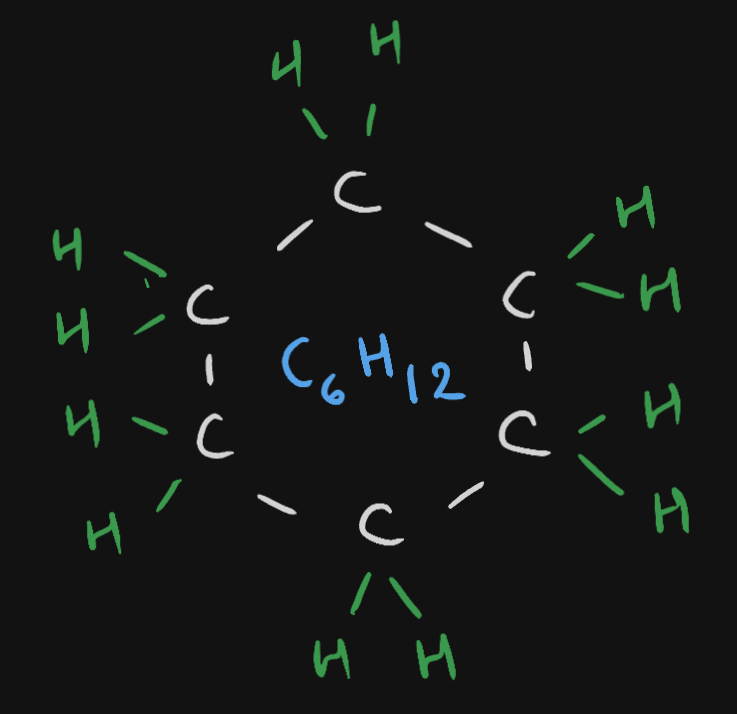
Benzene
The Benzene compound has a formula of 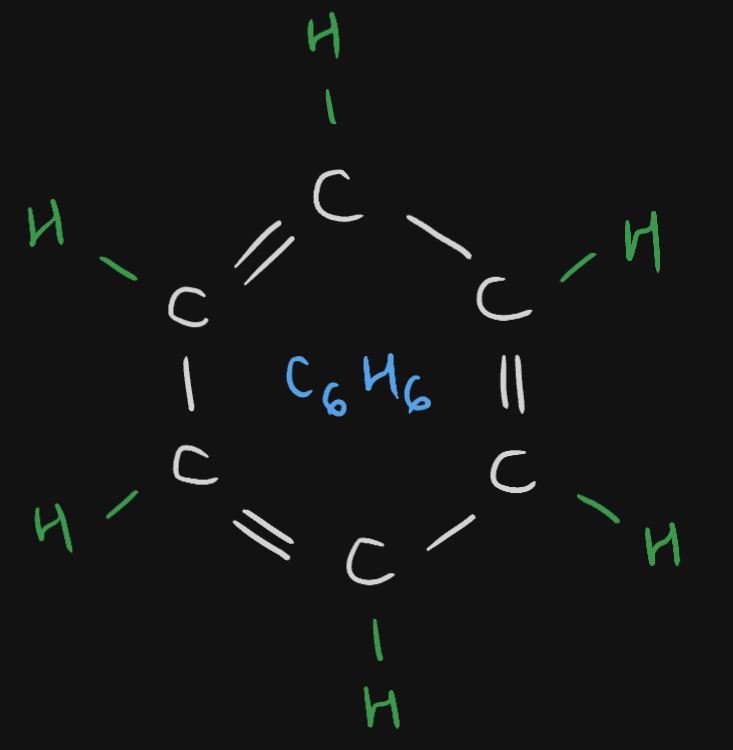
Backlinks
Carbon’s Specialties Hydrocarbons
Flashcards
What is catenation? ? Carbon has a tendency to form compounds with itself in the form of branches, chains and rings. This is called Catenation.
What are structural isomers? ? Same compound, same formula, same no. of atoms, but arranged differently.
Benzene compound has a formula of {{
Cyclohexane compound has a formula of {{