Definition
It is the reaction through which Brine (soluble
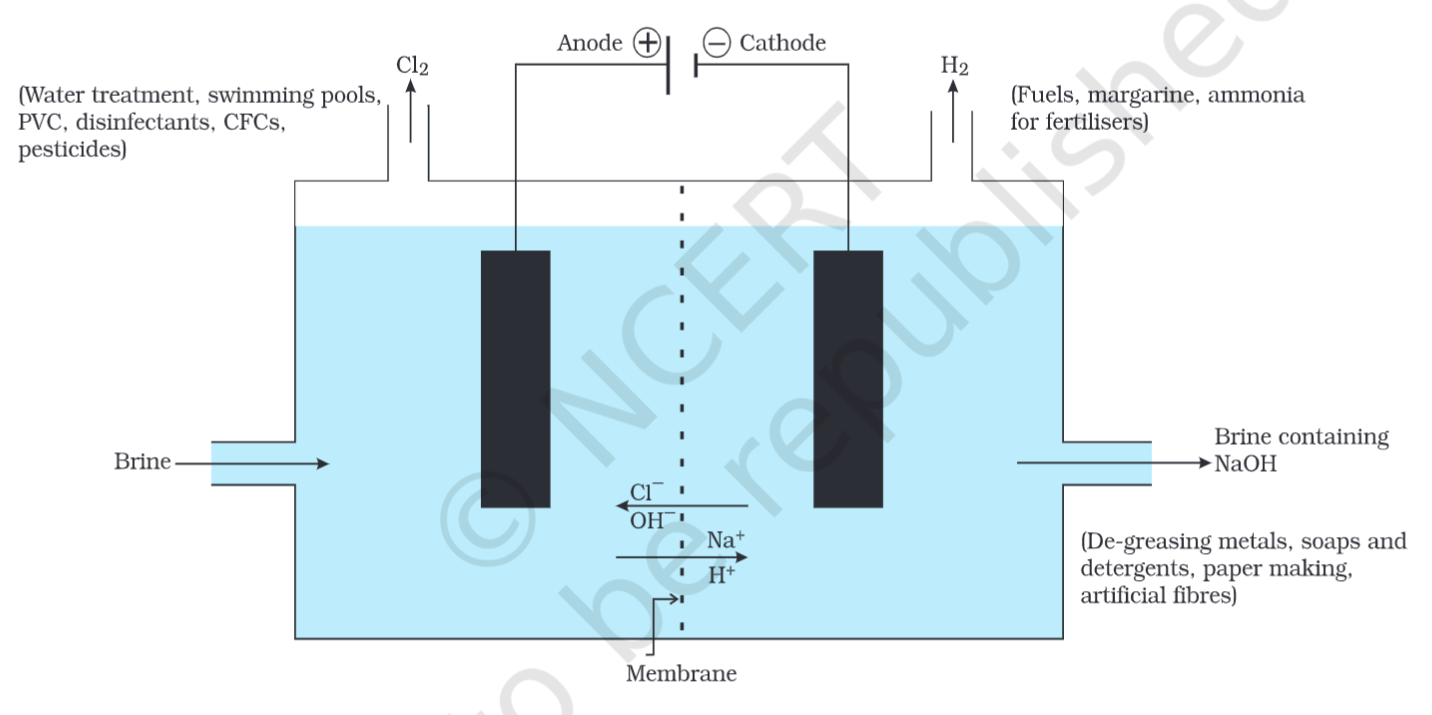
Products of Chlor-alkali Process
Sodium Hydroxide
- De-greasing of metal
- Paper Making
- Soaps, detergents
- Artificial Fibres
Hydrogen Gas
- In Making
(with Chlorine gas) - Used in making margarine and hydrogenation of oil
- In making fertilisers, etc.
Chlorine Gas
- Used in water treatment
- Used in making PVCs, CFCs, and pesticides
- Used as disinfectant
Backlinks
Flashcards
What is the chlor-alkali process?
?
It is the reaction through which Brine (soluble
Chemical Equation for the chlor-alkali process
?
What is brine? ? NaCl dissolved in water. Soluble NaCl
What are the by-products of the chlor-alkali process and what are their uses? ?
Sodium Hydroxide
- De-greasing of metal
- Paper Making
- Soaps, detergents
- Artificial Fibres
Hydrogen Gas
- In Making
(with Chlorine gas) - Used in making margarine and hydrogenation of oil
- In making fertilisers, etc.
Chlorine Gas
- Used in water treatment
- Used in making PVCs, CFCs, and pesticides
- Used as disinfectant